

This powder mixture is then crushed in a pellet press in order to form a pellet through which the beam of the spectrometer can pass.
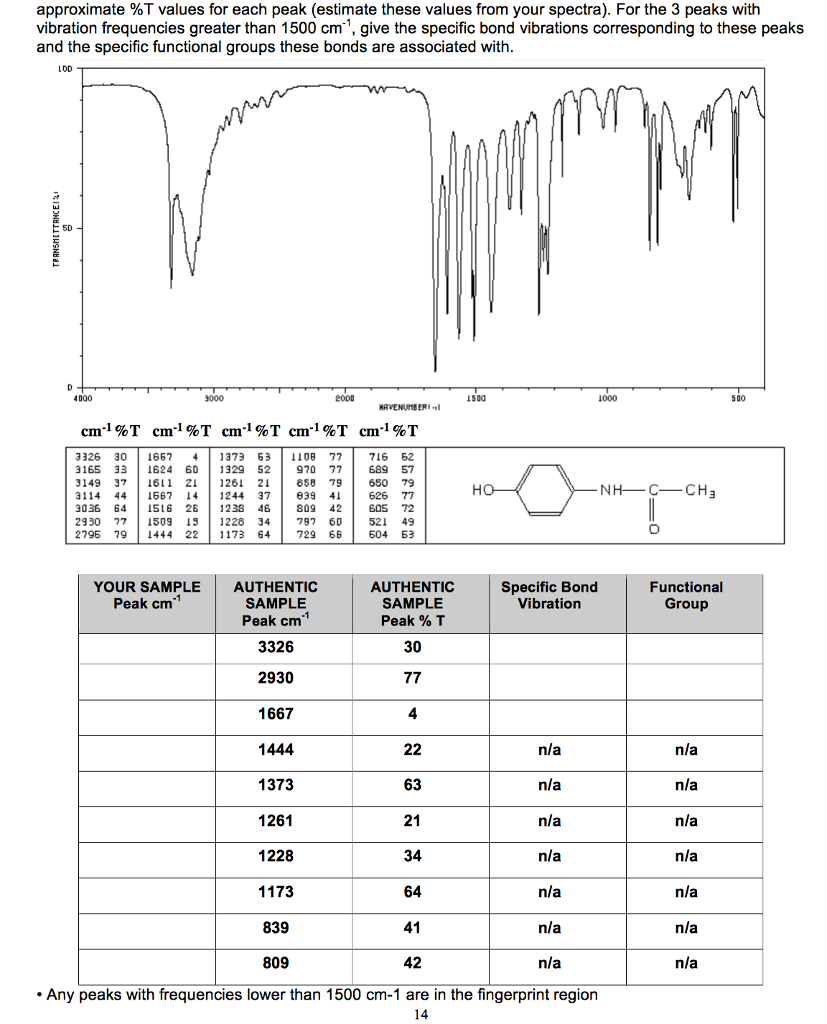
The second method is to mix a quantity of the sample with a specially purified salt (usually potassium bromide). If the solid can be induced to dissolve, or at least be crushed into a *very* fine powder, then the results will be good. The first is to crush the sample with a mulling agent in a marble pestle and mortar. Solid samples can be prepared in two major ways. The plates are obviously highly soluble in water, and so the sample and washing reagents and the like must be anhydrous (without water). The plates are transparent to the infrared light and will not introduce any lines onto the spectra. Liquid samples can be sandwiched between two plates of high purity salt (as in sodium chloride, or common salt). The technique has been used for the characterization of very complex mixtures however. Clear charts (or spectra) will be produced by samples with high levels of purity of one substance. This technique works almost exclusively on covalent bonds, and as such is of most use in organic chemistry. When looking at a chart for a substance, an experienced user can identify the substance from the information on the chart. By repeating this operation across a range of interest (usually no more than 4000-500cm -1), a chart can be built up. In order to measure a sample, a beam of monochromatic infrared light is passed through the sample, and the amount of energy absorbed is recorded. A useful flash animation of these can be found here ( ) Bonds can vibrate in six different ways, symmetrical and asymmetrical stretching, scissoring, rocking, wagging and twisting. Thus, the frequency of the vibrations can be associated with a particular bond type. These resonant frequencies are dependent on the length of the bond, and the mass of the atoms at either end of it. Infrared spectroscopy works because chemical bonds have specific frequencies at which they vibrate. Infrared spectroscopy (IR Spectroscopy) is a type of spectroscopy that uses the Infrared portion of the electromagnetic spectrum.Īs with all spectroscopic techniques, it can be used to investigate the composition of a sample. IR spectrum of a thin film of liquid ethanol.


 0 kommentar(er)
0 kommentar(er)
